- Nickel Atomic Number Electron Configuration
- Nickel Element Symbol
- Nickel Atomic Number
- What Is Nickel Atomic Number
The number of electrons in the outer energy level of a neutral atom of boron (atomic number 5) is: 3. What is the correct electron configuration for the lithium atom?: 1s2 2s1. What is the abbreviated electron configuration for nickel ( atomic number 28)?: Ar4s2 3d8. What is the element with the abbreviated electron configuration Kr5s2 4d8. Nickel-58 Please visit the Nickel element page for information specific to the chemical element of the periodic table. This is a list of the 118 chemical elements which have been identified as of 2021. A chemical element, often simply called an element, is a species of atoms which all have the same number of protons in their atomic nuclei (i.e., the same atomic number, or Z). The number of electrons are determined by the number of protons when the atom maintains its neutral charge. For elements to stay neutral, they must consist of an equal number of protons and electrons.Therefore the positively charged protons will balance with the negatively charged electrons. Atomic Structure of Nickel. Nickel-58 Ni CID 178157 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards.
The Element Nickel
Nickel Atomic Number Electron Configuration
[Click for Isotope Data]
Atomic Number: 28
Atomic Weight: 58.6934
Melting Point: 1728 K (1455°C or 2651°F)
Boiling Point: 3186 K (2913°C or 5275°F)
Density: 8.912 grams per cubic centimeter
Phase at Room Temperature: Solid
Element Classification: Metal
Period Number: 4
Group Number: 10
Group Name: none

What's in a name? From the German word Nickel, which means 'Old Nick,' a name for the devil. Also from the German word for the mineral niccolite, kupfernickel, which means 'Old Nick's copper.'
Say what? Nickel is pronounced as NIK-'l.
History and Uses:
Nickel was discovered by the Swedish chemist Axel Fredrik Cronstedt in the mineral niccolite (NiAs) in 1751. Today, most nickel is obtained from the mineral pentlandite (NiS·2FeS). Most of the world's supply of nickel is mined in the Sudbury region of Ontario, Canada. It is believed that this large deposit of nickel ore is a result of an ancient meteor impact.
Nickel is a hard, corrosion resistant metal. It can be electroplated onto other metals to form a protective coating. Finely divided nickel is used as a catalyst for the hydrogenation of vegetable oils. Adding nickel to glass gives it a green color. A single kilogram of nickel can be drawn into 300 kilometers of wire. Nickel is also used to manufacture some types of coins and batteries.
Nickel is alloyed with other metals to improve their strength and resistance to corrosion. Nickel is alloyed with steel to make armor plate, vaults and machine parts. It is alloyed with copper to make pipes that are used in desalination plants. Very powerful permanent magnets, known as Alnico magnets, can be made from an alloy of aluminum, nickel, cobalt and iron.
Estimated Crustal Abundance: 8.4×101 milligrams per kilogram
Estimated Oceanic Abundance: 5.6×10-4 milligrams per liter
Number of Stable Isotopes: 5 (View all isotope data)
Ionization Energy: 7.640 eV
Oxidation States: +3, +2
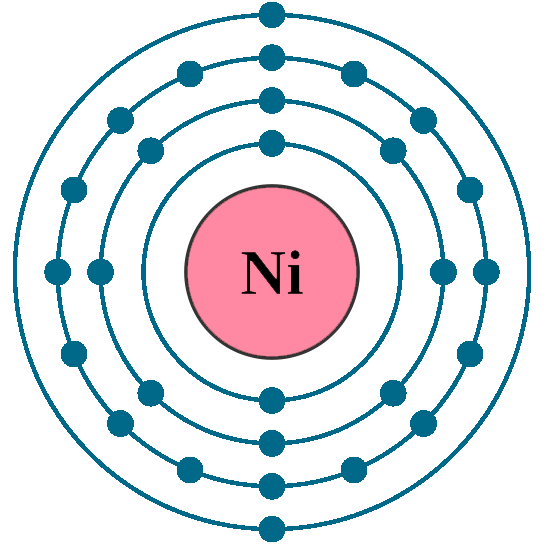
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 3d8 | |
4s2 |
Nickel Element Symbol

For questions about this page, please contact Steve Gagnon.
The Element Nickel
[Click for Isotope Data]
Nickel Atomic Number
Atomic Number: 28
Atomic Weight: 58.6934
Melting Point: 1728 K (1455°C or 2651°F)
Boiling Point: 3186 K (2913°C or 5275°F)
Density: 8.912 grams per cubic centimeter
Phase at Room Temperature: Solid
What Is Nickel Atomic Number
Element Classification: Metal
Period Number: 4
Group Number: 10
Free download midi karaoke dangdut koplo terbaru. Group Name: none
What's in a name? From the German word Nickel, which means 'Old Nick,' a name for the devil. Also from the German word for the mineral niccolite, kupfernickel, which means 'Old Nick's copper.'
Say what? Nickel is pronounced as NIK-'l.
History and Uses:
Nickel was discovered by the Swedish chemist Axel Fredrik Cronstedt in the mineral niccolite (NiAs) in 1751. Today, most nickel is obtained from the mineral pentlandite (NiS·2FeS). Most of the world's supply of nickel is mined in the Sudbury region of Ontario, Canada. It is believed that this large deposit of nickel ore is a result of an ancient meteor impact.
Nickel is a hard, corrosion resistant metal. It can be electroplated onto other metals to form a protective coating. Finely divided nickel is used as a catalyst for the hydrogenation of vegetable oils. Adding nickel to glass gives it a green color. A single kilogram of nickel can be drawn into 300 kilometers of wire. Nickel is also used to manufacture some types of coins and batteries.
Nickel is alloyed with other metals to improve their strength and resistance to corrosion. Nickel is alloyed with steel to make armor plate, vaults and machine parts. It is alloyed with copper to make pipes that are used in desalination plants. Very powerful permanent magnets, known as Alnico magnets, can be made from an alloy of aluminum, nickel, cobalt and iron.
Estimated Crustal Abundance: 8.4×101 milligrams per kilogram
Estimated Oceanic Abundance: 5.6×10-4 milligrams per liter
Number of Stable Isotopes: 5 (View all isotope data)
Ionization Energy: 7.640 eV
Oxidation States: +3, +2
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 3d8 | |
4s2 |
For questions about this page, please contact Steve Gagnon.
